
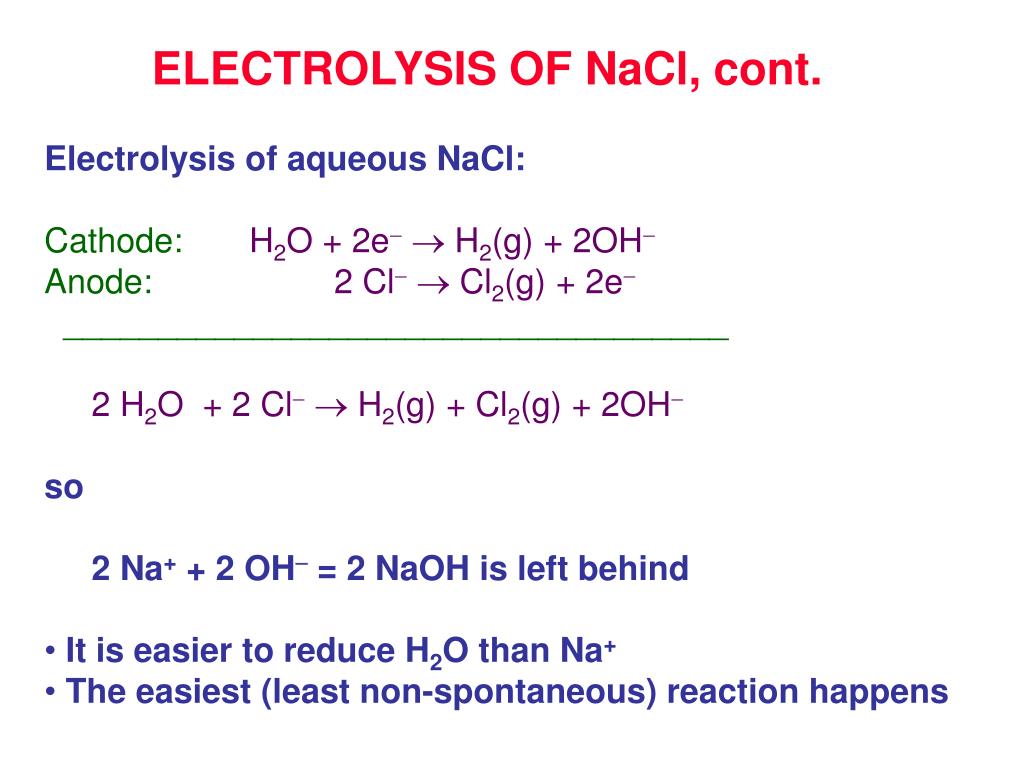
Issue of viability important but at this temperature molten sodium formsĪ thick fog inside the reaction vessel which is difficult to recover. Cathode reaction: Cu 2+ (aq) + 2e- Cu(s) Anode reaction: 2H 2 O(l) O 2 (g) + 4H + (aq) + 4e-With carbon (graphite) electrodes, the oxygen usually reacts with the anode to form CO 2. Requires a significant amount of energy to be supplied. Now sodium chloride melts at approximately 800☌ this This compound is canonicalized and has one covalently bonded unit. The number of hydrogen bond acceptors equals to zero and the number of hydrogen bond donors equals to zero. The highĬurrent passing through the cell provides sufficient heat to keep the The exact mass and the monoisotopic mass of Cupric chloride is 132.867 g/mol.
Silver can be electroplated at the cathode of an electrolysis cell by the. Molten mixture and is collected in a water tight storage vessel. reaction, that reaction is not a redox reaction. An iron mesh screen is used to preventĬhlorine gas, formed at the anode, from coming into contact with sodium The cell consists of a central carbon anode surroundedīy a cylindrical iron cathode. The reaction mechanism of electrolytic reduction of SiO2 at a liquid Zn cathode in molten CaCl2 was investigated with the aim of establishing a new production process of solar-grade Si. The reaction mechanism of electrolytic reduction of SiO2 at a liquid Zn cathode in molten CaCl2 was investigated with the aim of establishing a new production process of solar-grade Si.
#CATHODE REACTION IN ELECTROLYTSIS OF CUCL2 FULL#
The sea is full of it and we can easily get to it. 8.27 Predict the products of electrolysis in each of the following: (iv) An aqueous solution of CuCl2 with platinum electrodes. Niether sodium nor chlorine, however, occur naturally in nature, but we do have a plentyfull source in the form of salt (NaCl). Sodium metal and chlorine gas are starting materials for the inudstrial production of a range of substances ranging from plastics to sodium fluoride found in toothpaste.


 0 kommentar(er)
0 kommentar(er)
